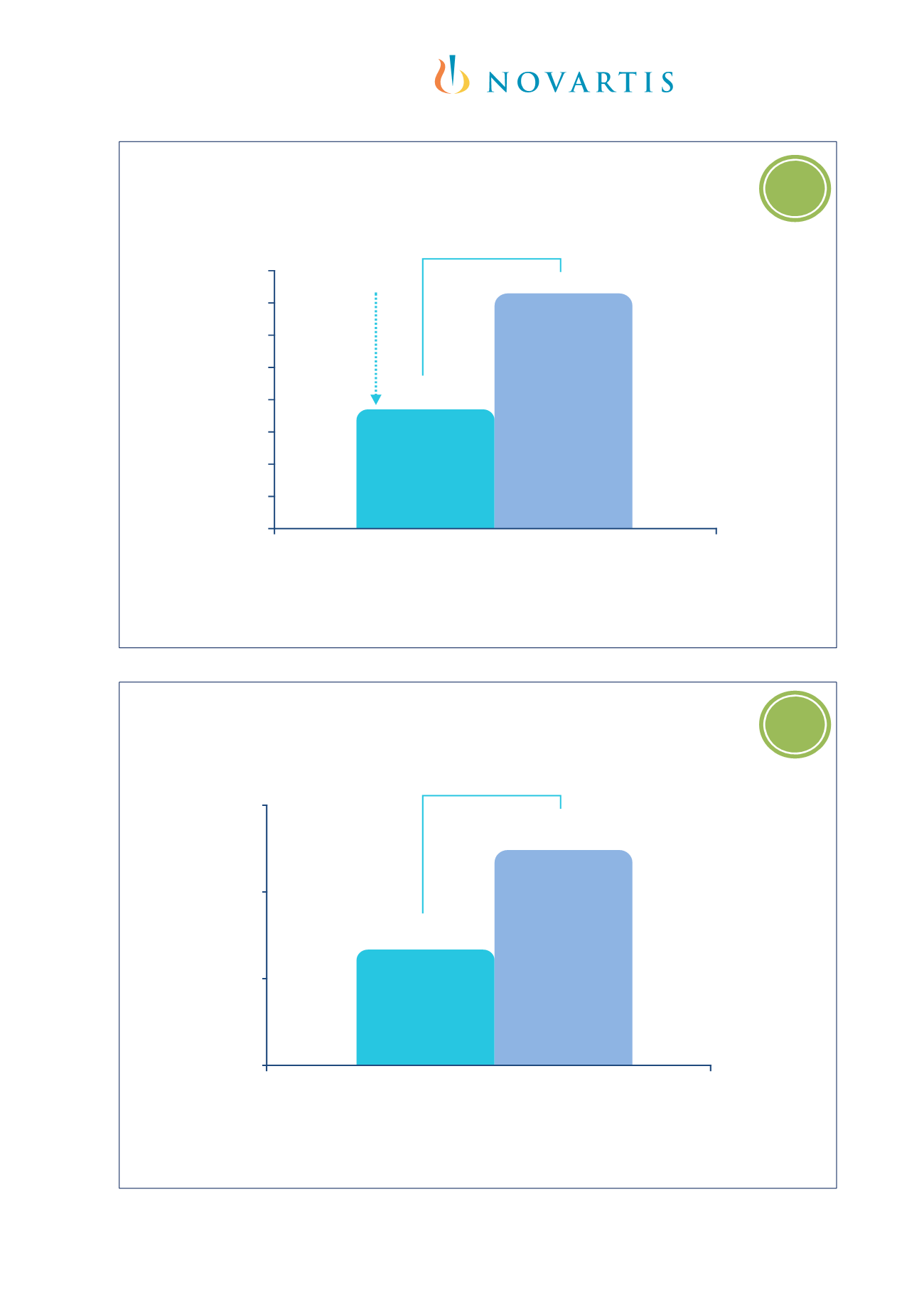
185
0,27
0,50
0
0,2
0,4
0,6
Severe exacerbations* over 52 weeks
*Requiring treatment with systemic corticosteroids and where the
patients had PEF or FEV
1
<60% of personal best.
Kulus M,
et al. Clin Exp Allergy
2009;39:1953 (abstract).
Severe exacerbation rate
Omalizumab
(n=159)
Placebo
(n=76)
p=0.084
IA05-EU
Consistent reduction in
exacerbations
*
in severe
population
*Defined as worsening of asthma symptoms requiring doubling of
baseline ICS dose and/or treatment with rescue systemic
corticosteroids for >3 days.
Kulus M,
et al. Curr Med Res Opin
2010;26:1285–93.
0,73
1,44
Omalizumab
(n=159)
Placebo
(n=76)
Clinically significant exacerbation rate
0.0
0.2
0.4
0.6
0.8
1.0
1.2
1.4
1.6
p<0.001
RRR
50%
IA05-EU
Simposium


