183
Omalizumab in children with allergic
asthma
1
LanierB,
etal
.
JAllergyClinImmunol
2009;124:1210–6;
2
KulusM,
etal
.
CurrMedResOpin
2010;26:1285–93;
3
MilgromH,
etal
.
Pediatrics
2001;108:e36;
4
BusseW,
etal
.
NE
n
glJMed
2011;364:1005–15
IA05
1
Omalizumab reduced
exacerbations
over 52 weeks in children
with
severe
allergic asthma
IA05-EU
2
Moderate‐to‐severe
(well controlled)
N=334
Steroid sparing
28 Weeks
010
3
Persistent asthma
(any severity)
N=419
Symptom‐free days
60 Weeks
ICATA
4
Moderate‐to‐severe
N=628
Exacerbations
52 Weeks
0.45
0.64
0,32
0,71
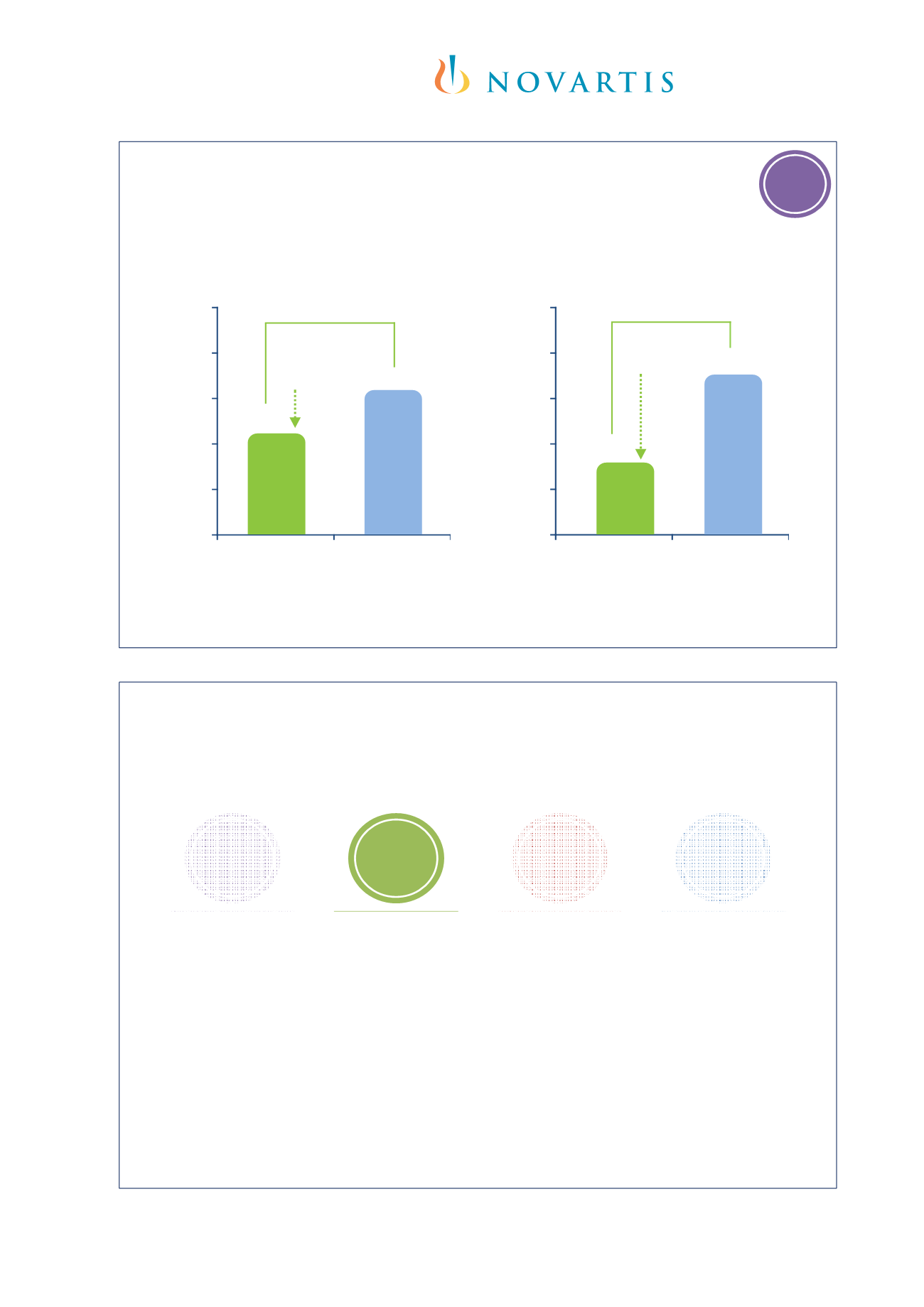
Clinically significant exacerbations*
reduced during both study phases
Fixed-steroid phase (Week 0
24) – primary analysis
1
Omalizumab
(n=384)
Placebo
(n=192)
Clinically significant exacerbation rate
p=0.007
1.0
0.8
0.6
0.4
0.2
0.0
Adjustable-steroid phase (Week 24
52)
2
Clinically significant exacerbation rate
p<0.001
Omalizumab
(n=384)
Placebo
(n=192)
1.0
0.8
0.6
0.4
0.2
0.0
RRR
31%
RRR
54%
IA05
Simposium


