181
Baseline characteristics
All patients were receiving ICS
Reduced lung function
High ICS use at baseline
High baseline IgE levels
IA05
1,2
010
3
ICATA
4
Gender
(%)
29.8%
female
41%
female
IgE
(IU/mL)
469.7
348
Age
(years)
9.4
10.9
8.6
32.3%
female
Not
available
Omalizumab in children with allergic
asthma
ICS = inhaled corticosteroids.
1
Lanier B,
et al
.
J Allergy Clin Immunol
2009;124:1210–6;
2
Kulus M,
et al
.
Curr Med Res Opin
2010;26:1285–93;
3
Milgrom H,
et al
.
Pediatrics
2001;108:e36;
4
Busse W,
et al
.
N E
n
gl J Med
2011;364:1005–15.
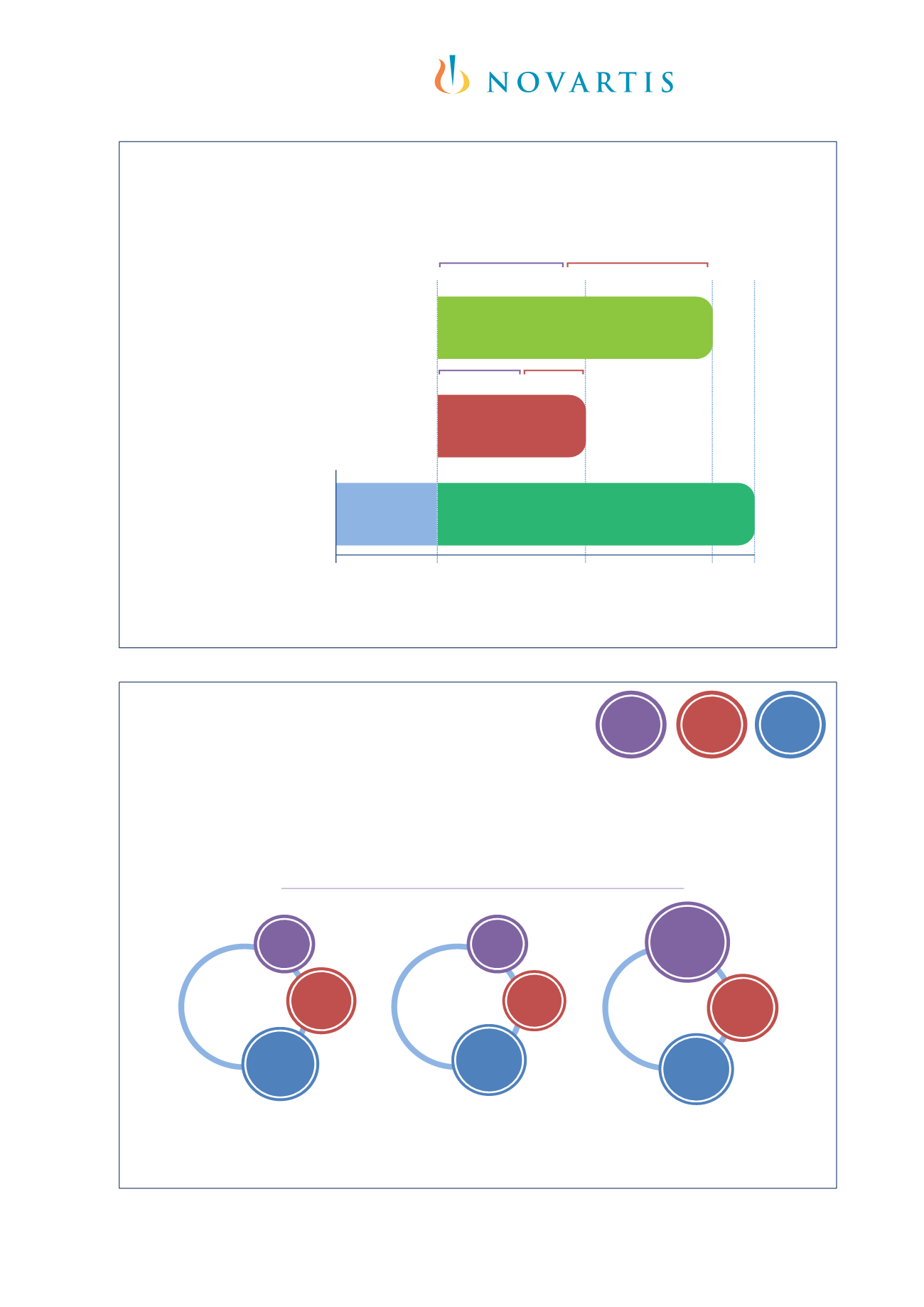
Double‐Blind Trials
Moderate‐to‐severe asthma
Age 6 to <12 years
Medium‐/high‐dose ICS
Controlled trial
Persistent allergic asthma
or evidence of uncontrolled
disease
Age 6–20 years
Inner city
4-week run-in
Randomization
60
weeks
52
weeks
28
weeks
Omalizumab (n=421)
Placebo (n=207)
Omalizumab (n=225)
Placebo (n=109)
Omalizumab (n=208)
Placebo (n=211)
Fixed steroid phase Adjustable steroid phase
ICATA
4
STUDY 010
3
IA05
1,2
well-controlled asthma
Simposium


